1. Brazeability
The brazing property of aluminum and aluminum alloys is poor, mainly because the oxide film on the surface is difficult to remove. Aluminum has great affinity for oxygen. It is easy to form a dense, stable and high melting point oxide film Al2O3 on the surface. At the same time, aluminum alloys containing magnesium will also form a very stable oxide film MgO. They will seriously hinder the wetting and spreading of solder. And hard to remove. During brazing, the brazing process can be carried out only with proper flux.
Secondly, the operation of aluminum and aluminum alloy brazing is difficult. The melting point of aluminum and aluminum alloy is not much different from that of the brazing filler metal used. The optional temperature range for brazing is very narrow. A little improper temperature control is easy to cause overheating or even melting of the base metal, making the brazing process difficult. Some aluminum alloys strengthened by heat treatment will also cause softening phenomena such as over aging or annealing due to brazing heating, which will reduce the properties of brazed joints. During flame brazing, it is difficult to judge the temperature because the color of aluminum alloy does not change during heating, which also increases the requirements for the operator’s operation level.
Moreover, the corrosion resistance of aluminum and aluminum alloy brazed joints is easily affected by filler metals and fluxes. The electrode potential of aluminum and aluminum alloy is quite different from that of solder, which reduces the corrosion resistance of the joint, especially for the soft soldering joint. In addition, most of the fluxes used in the brazing of aluminum and aluminum alloys have strong corrosivity. Even if they are cleaned after brazing, the influence of fluxes on the corrosion resistance of joints will not be completely eliminated.
2. Brazing material
(1) Brazing of aluminum and aluminum alloys is a rarely used method, because the composition and electrode potential of brazing filler metal and base metal are very different, which is easy to cause electrochemical corrosion of the joint. The soft soldering mainly adopts zinc based solder and tin lead solder, which can be divided into low temperature solder (150 ~ 260 ℃), medium temperature solder (260 ~ 370 ℃) and high temperature solder (370 ~ 430 ℃) according to the temperature range. When tin lead solder is used and copper or nickel is pre plated on the aluminum surface for brazing, corrosion at the joint interface can be prevented, so as to improve the corrosion resistance of the joint.
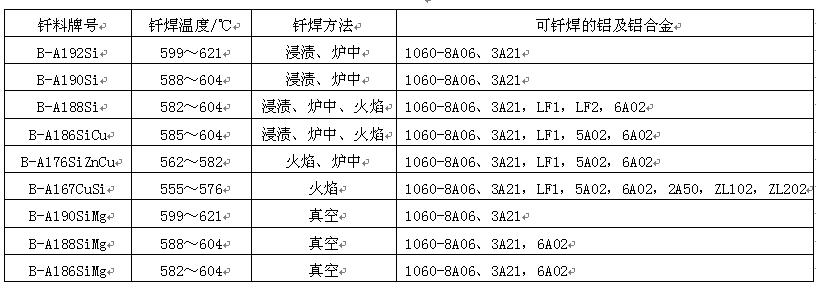
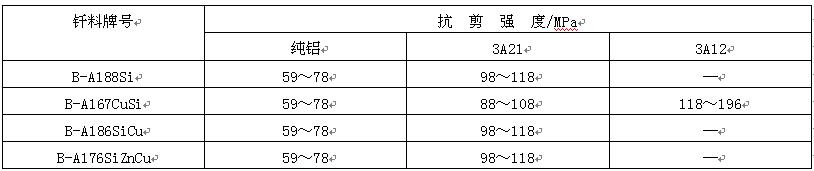
Brazing of aluminum and aluminum alloys is widely used, such as filter guide, evaporator, radiator and other components. Only aluminum based filler metals can be used for brazing of aluminum and aluminum alloys, among which aluminum silicon filler metals are the most widely used. The specific scope of application and the shear strength of brazed joints are shown in Table 8 and table 9 respectively. However, the melting point of this solder is close to that of the base metal, so the heating temperature should be strictly and accurately controlled during brazing to avoid overheating or even melting of the base metal.
Table 8 application scope of brazing filler metals for aluminum and aluminum alloys
Table 9 shear strength of aluminum and aluminum alloy joints brazed with aluminum silicon filler metals
Aluminum silicon solder is usually supplied in the form of powder, paste, wire or sheet. In some cases, solder composite plates with aluminum as the core and aluminum silicon solder as the cladding are used. This kind of solder composite plate is made by hydraulic method and is often used as a part of brazing components. During brazing, the brazing filler metal on the composite plate melts and flows under the action of capillary and gravity to fill the joint gap.
(2) Flux and shielding gas for aluminum and aluminum alloy brazing, special flux is often used to remove the film. The organic flux based on triethanolamine, such as fs204, is used with low-temperature soft solder. The advantage of this flux is that it has little corrosion effect on the base metal, but it will produce a large amount of gas, which will affect the wetting and caulking of the solder. The reactive flux based on zinc chloride, such as fs203 and fs220a, is used with medium temperature and high temperature soft solder. The reactive flux is highly corrosive, and its residue must be removed after brazing.
At present, the brazing of aluminum and aluminum alloys is still dominated by flux film removal. The brazing flux used includes chloride based flux and fluoride based flux. Chloride based flux has strong ability to remove oxide film and good fluidity, but it has a great corrosive effect on the base metal. Its residue must be completely removed after brazing. Fluoride based flux is a new type of flux, which has good film removal effect and no corrosion to base metal. However, it has high melting point and poor thermal stability, and can only be used with aluminum silicon solder.
When brazing aluminum and aluminum alloys, vacuum, neutral or inert atmosphere is often used. When vacuum brazing is used, the vacuum degree shall generally reach the order of 10-3pa. When nitrogen or argon gas is used for protection, its purity must be very high, and the dew point must be lower than -40 ℃
3. Brazing technology
Brazing of aluminum and aluminum alloys has high requirements for the cleaning of workpiece surface. In order to obtain good quality, the oil stain and oxide film on the surface must be removed before brazing. Remove the oil stain on the surface with Na2CO3 aqueous solution at a temperature of 60 ~ 70 ℃ for 5 ~ 10min, and then rinse with clean water; The surface oxide film can be removed by etching with NaOH aqueous solution at a temperature of 20 ~ 40 ℃ for 2 ~ 4min, and then washed with hot water; After removing the oil stain and oxide film on the surface, the workpiece shall be treated with HNO3 aqueous solution for gloss for 2 ~ 5min, then cleaned in running water and finally dried. The workpiece treated by these methods shall not be touched or contaminated with other dirt, and shall be brazed within 6 ~ 8h. It is better to braze immediately if possible.
The brazing methods of aluminum and aluminum alloys mainly include flame brazing, soldering iron brazing and furnace brazing. These methods generally use flux in brazing, and have strict requirements on heating temperature and holding time. During flame brazing and soldering iron brazing, avoid heating the flux directly by the heat source to prevent the flux from overheating and failure. Since aluminum can be dissolved in soft solder with high zinc content, heating should be stopped once the joint is formed to avoid base metal corrosion. In some cases, the brazing of aluminum and aluminum alloys sometimes does not use flux, but uses ultrasonic or scraping methods to remove the film. When using scraping to remove the film for brazing, first heat the workpiece to the brazing temperature, and then scrape the brazing part of the workpiece with the end of the solder rod (or scraping tool). While breaking the surface oxide film, the end of the solder will melt and wet the base metal.
Brazing methods of aluminum and aluminum alloys mainly include flame brazing, furnace brazing, dip brazing, vacuum brazing and gas shielded brazing. Flame brazing is mostly used for small workpieces and single piece production. In order to avoid the failure of the flux due to the contact between the impurities in acetylene and the flux when using oxyacetylene flame, it is appropriate to use gasoline compressed air flame with slight reducibility to prevent the oxidation of the base metal. During specific brazing, the brazing flux and filler metal can be placed at the brazed place in advance and heated at the same time with the workpiece; The workpiece can also be heated to the brazing temperature first, and then the solder dipped with flux can be sent to the brazing position; After the flux and filler metal are melted, the heating flame shall be removed slowly after the filler metal is evenly filled.
When brazing aluminum and aluminum alloy in an air furnace, the brazing filler metal shall be preset, and the brazing flux shall be melted in distilled water to prepare a thick solution with a concentration of 50% ~ 75%, and then coated or sprayed on the brazing surface. An appropriate amount of powder brazing flux can also be covered on the brazing filler metal and brazing surface, and then the assembled weldment shall be placed in the furnace for heating brazing. In order to prevent the base metal from overheating or even melting, the heating temperature must be strictly controlled.
Paste or foil solder is generally used for dip brazing of aluminum and aluminum alloys. The assembled workpiece shall be preheated before brazing to make its temperature close to the brazing temperature, and then immersed in brazing flux for brazing. During brazing, the brazing temperature and brazing time shall be strictly controlled. If the temperature is too high, the base metal is easy to dissolve and the solder is easy to be lost; If the temperature is too low, the solder is not melted enough, and the brazing rate decreases. The brazing temperature shall be determined according to the type and size of the base metal, the composition and melting point of the filler metal, and is generally between the liquidus temperature of the filler metal and the solidus temperature of the base metal. The dipping time of the workpiece in the flux bath must ensure that the solder can fully melt and flow, and the supporting time should not be too long. Otherwise, the silicon element in the solder may diffuse into the base metal, making the base metal near the seam brittle.
In vacuum brazing of aluminum and aluminum alloys, metal operating activators are often used to modify the surface oxide film of aluminum and ensure the wetting and spreading of solder. Magnesium can be directly used on the workpiece in the form of particles, or introduced into the brazing zone in the form of steam, or magnesium can be added to the aluminum silicon solder as an alloy element. For the workpiece with complex structure, in order to ensure the full effect of magnesium vapor on the base metal and improve the brazing quality, local shielding process measures are often taken, that is, the workpiece is first placed in a stainless steel box (commonly known as the process box), and then placed in a vacuum furnace for heating brazing. Vacuum Brazed Aluminum and aluminum alloy joints have smooth surface and dense brazed joints, and do not need to be cleaned after brazing; However, the vacuum brazing equipment is expensive, and the magnesium vapor pollutes the furnace seriously, so it needs to be cleaned and maintained frequently.
When brazing aluminum and aluminum alloys in neutral or inert atmosphere, magnesium activator or flux can be used to remove the film. When magnesium activator is used to remove the film, the amount of magnesium required is much lower than that of vacuum brazing. Generally, w (mg) is about 0.2% ~ 0.5%. When the content of magnesium is high, the quality of the joint will be reduced. NOCOLOK brazing method using fluoride flux and nitrogen protection is a new method developed rapidly in recent years. Since the residue of fluoride flux does not absorb moisture and is not corrosive to aluminum, the process of removing flux residue after brazing can be omitted. Under the protection of nitrogen, only a small amount of fluoride flux needs to be coated, the filler metal can well wet the base metal, and it is easy to obtain high-quality brazed joints. At present, this NOCOLOK brazing method has been used in the mass production of aluminum radiator and other components.
For aluminum and aluminum alloy brazed with flux other than fluoride flux, the flux residue must be completely removed after brazing. The residue of organic brazing flux for aluminum can be washed with organic solutions such as methanol and trichloroethylene, neutralized with sodium hydroxide aqueous solution, and finally cleaned with hot and cold water. Chloride is the residue of brazing flux for aluminum, which can be removed according to the following methods; First, soak in hot water at 60 ~ 80 ℃ for 10min, carefully clean the residue on the brazed joint with a brush, and clean it with cold water; Then soak it in 15% nitric acid aqueous solution for 30min, and finally rinse it with cold water.
Post time: Jun-13-2022