(1) Brazing characteristics the problems involved in graphite and diamond polycrystalline brazing are very similar to those encountered in ceramic brazing. Compared with metal, solder is difficult to wet graphite and diamond polycrystalline materials, and its coefficient of thermal expansion is very different from that of general structural materials. The two are heated directly in air, and oxidation or carbonization will occur when the temperature exceeds 400 ℃. Therefore, vacuum brazing shall be adopted, and the vacuum degree shall not be less than 10-1pa. Because the strength of both is not high, if there is thermal stress during brazing, cracks may occur. Try to select brazing filler metal with low coefficient of thermal expansion and strictly control the cooling rate. Since the surface of such materials is not easy to be wetted by ordinary brazing filler metals, a layer of 2.5 ~ 12.5um thick W, Mo and other elements can be deposited on the surface of graphite and diamond polycrystalline materials by surface modification (vacuum coating, ion sputtering, plasma spraying and other methods) before brazing and form corresponding carbides with them, or high activity brazing filler metals can be used.
Graphite and diamond have many grades, which differ in particle size, density, purity and other aspects, and have different brazing characteristics. In addition, if the temperature of polycrystalline diamond materials exceeds 1000 ℃, the polycrystalline wear ratio begins to decrease, and the wear ratio decreases by more than 50% when the temperature exceeds 1200 ℃. Therefore, when vacuum brazing diamond, the brazing temperature must be controlled below 1200 ℃, and the vacuum degree shall not be less than 5 × 10-2Pa.
(2) The choice of brazing filler metal is mainly based on the use and surface processing. When used as a heat-resistant material, the brazing filler metal with high brazing temperature and good heat resistance shall be selected; For chemical corrosion-resistant materials, brazing filler metals with low brazing temperature and good corrosion resistance are selected. For the graphite after surface metallization treatment, pure copper solder with high ductility and good corrosion resistance can be used. Silver based and copper based active solder have good wettability and fluidity to graphite and diamond, but the service temperature of brazed joint is difficult to exceed 400 ℃. For graphite components and diamond tools used between 400 ℃ and 800 ℃, gold base, palladium base, manganese base or titanium base filler metals are usually used. For joints used between 800 ℃ and 1000 ℃, nickel based or drill based filler metals shall be used. When graphite components are used above 1000 ℃, pure metal filler metals (Ni, PD, Ti) or alloy filler metals containing molybdenum, Mo, Ta and other elements that can form carbides with carbon can be used.
For graphite or diamond without surface treatment, the active filler metals in table 16 can be used for direct brazing. Most of these filler metals are titanium based binary or ternary alloys. Pure titanium reacts strongly with graphite, which can form a very thick carbide layer, and its linear expansion coefficient is quite different from that of graphite, which is easy to produce cracks, so it can not be used as solder. The addition of Cr and Ni to Ti can reduce the melting point and improve the wettability with ceramics. Ti is a ternary alloy, mainly composed of Ti Zr, with the addition of TA, Nb and other elements. It has a low coefficient of linear expansion, which can reduce the brazing stress. The ternary alloy mainly composed of Ti Cu is suitable for the brazing of graphite and steel, and the joint has high corrosion resistance.
Table 16 brazing filler metals for direct brazing of graphite and diamond
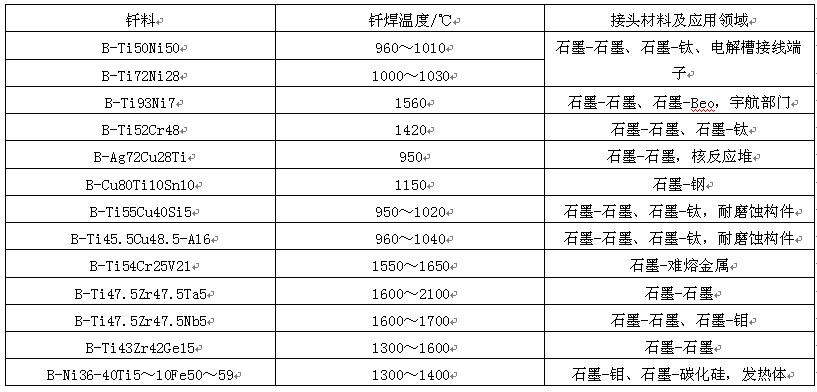
(3) Brazing process the brazing methods of graphite can be divided into two categories, one is brazing after surface metallization, and the other is brazing without surface treatment. No matter which method is used, the weldment shall be pretreated before assembly, and the surface contaminants of graphite materials shall be wiped clean with alcohol or acetone. In case of surface metallization brazing, a layer of Ni, Cu or a layer of Ti, Zr or molybdenum disilicide shall be plated on the graphite surface by plasma spraying, and then copper based filler metal or silver based filler metal shall be used for brazing. Direct brazing with active solder is the most widely used method at present. The brazing temperature can be selected according to the solder provided in table 16. The solder can be clamped in the middle of the brazed joint or near one end. When brazing with a metal with a large coefficient of thermal expansion, Mo or Ti with a certain thickness can be used as the intermediate buffer layer. The transition layer can produce plastic deformation during brazing heating, absorb thermal stress and avoid graphite cracking. For example, Mo is used as the transition joint for vacuum brazing of graphite and hastelloyn components. B-pd60ni35cr5 solder with good resistance to molten salt corrosion and radiation is used. The brazing temperature is 1260 ℃ and the temperature is kept for 10min.
Natural diamond can be directly brazed with b-ag68.8cu16.7ti4.5, b-ag66cu26ti8 and other active solders. The brazing shall be carried out under vacuum or low argon protection. The brazing temperature should not exceed 850 ℃, and a faster heating rate should be selected. The holding time at the brazing temperature should not be too long (generally about 10s) to avoid the formation of a continuous tic layer at the interface. When brazing diamond and alloy steel, plastic interlayer or low expansion alloy layer should be added for transition to prevent the damage of diamond grains caused by excessive thermal stress. The turning tool or boring tool for ultra precision machining is manufactured by brazing process, which brazes 20 ~ 100mg small particle diamond onto the steel body, and the joint strength of the brazing joint reaches 200 ~ 250mpa
Polycrystalline diamond can be brazed by flame, high frequency or vacuum. High frequency brazing or flame brazing shall be adopted for diamond circular saw blade cutting metal or stone. Ag Cu Ti active brazing filler metal with low melting point shall be selected. The brazing temperature shall be controlled below 850 ℃, the heating time shall not be too long, and a slow cooling rate shall be adopted. Polycrystalline diamond bits used in petroleum and geological drilling have poor working conditions and bear huge impact loads. Nickel based brazing filler metal can be selected and pure copper foil can be used as the interlayer for vacuum brazing. For example, 350 ~ 400 capsules Ф 4.5 ~ 4.5mm columnar polycrystalline diamond is brazed into the perforations of 35CrMo or 40CrNiMo steel to form cutting teeth. Vacuum brazing is adopted, and the vacuum degree is not less than 5 × 10-2Pa, the brazing temperature is 1020 ± 5 ℃, the holding time is 20 ± 2min, and the shear strength of the brazing joint is greater than 200mpa
During brazing, the self weight of the weldment shall be used for assembly and positioning as much as possible to make the metal part press the graphite or polycrystalline material at the upper part. When using the fixture for positioning, the fixture material shall be the material with the thermal expansion coefficient similar to that of the weldment.
Post time: Jun-13-2022