Brazing of stainless steel
1. Brazeability
The primary problem in stainless steel brazing is that the oxide film on the surface seriously affects the wetting and spreading of solder. Various stainless steels contain a considerable amount of Cr, and some also contain Ni, Ti, Mn, Mo, Nb and other elements, which can form a variety of oxides or even composite oxides on the surface. Among them, the oxides Cr2O3 and TiO2 of Cr and Ti are quite stable and difficult to remove. When brazing in air, active flux must be used to remove them; When brazing in protective atmosphere, the oxide film can be reduced only in high purity atmosphere with low dew point and high enough temperature; In vacuum brazing, it is necessary to have enough vacuum and enough temperature to achieve good brazing effect.
Another problem of stainless steel brazing is that the heating temperature has a serious effect on the structure of the base metal. The brazing heating temperature of austenitic stainless steel shall not be higher than 1150 ℃, otherwise the grain will grow seriously; If austenitic stainless steel does not contain stable element Ti or Nb and has high carbon content, brazing within the sensitization temperature (500 ~ 850 ℃) shall also be avoided. To prevent the corrosion resistance from decreasing due to the precipitation of chromium carbide. The selection of brazing temperature for martensitic stainless steel is more strict. One is to match the brazing temperature with the quenching temperature, so as to combine the brazing process with the heat treatment process; The other is that the brazing temperature should be lower than the tempering temperature to prevent the base metal from softening during brazing. The brazing temperature selection principle of precipitation hardening stainless steel is the same as that of martensitic stainless steel, that is, the brazing temperature must match the heat treatment system to obtain the best mechanical properties.
In addition to the above two main problems, there is a tendency of stress cracking when brazing austenitic stainless steel, especially when brazing with copper zinc filler metal. In order to avoid stress cracking, the workpiece shall be stress relieved annealed before brazing, and the workpiece shall be uniformly heated during brazing.
2. Brazing material
(1) According to the use requirements of stainless steel weldments, the commonly used brazing filler metals for stainless steel weldments include Tin Lead brazing filler metal, silver based brazing filler metal, copper based brazing filler metal, manganese based brazing filler metal, nickel based brazing filler metal and precious metal brazing filler metal.
Tin lead solder is mainly used for stainless steel soldering, and it is suitable to have high tin content. The higher the tin content of the solder, the better its wettability on stainless steel. The shear strength of 1Cr18Ni9Ti stainless steel joints brazed with several common tin lead solders is listed in Table 3. Due to the low strength of the joints, they are only used for brazing parts with small bearing capacity.
Table 3 shear strength of 1Cr18Ni9Ti stainless steel joint brazed with tin lead solder

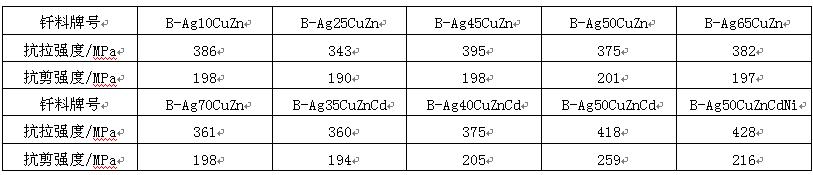
Silver based filler metals are the most commonly used filler metals for brazing stainless steel. Among them, silver copper zinc and silver copper zinc cadmium filler metals are most widely used because the brazing temperature has little effect on the properties of the base metal. The strength of ICr18Ni9Ti stainless steel joints brazed with several common silver based solders is listed in Table 4. The stainless steel joints brazed with silver based solders are rarely used in highly corrosive media, and the working temperature of the joints generally does not exceed 300 ℃. When brazing stainless steel without nickel, in order to prevent corrosion of brazed joint in humid environment, brazing filler metal with more nickel shall be used, such as b-ag50cuzncdni. When brazing martensitic stainless steel, in order to prevent softening of base metal, brazing filler metal with brazing temperature not exceeding 650 ℃ shall be used, such as b-ag40cuzncd. When brazing stainless steel in protective atmosphere, in order to remove the oxide film on the surface, lithium containing self brazing flux can be used, such as b-ag92culi and b-ag72culi. When brazing stainless steel in vacuum, in order to make the filler metal still have good wettability when it does not contain elements such as Zn and CD that are easy to evaporate, the silver filler metal containing elements such as Mn, Ni and RD can be selected.
Table 4 strength of ICr18Ni9Ti stainless steel joint brazed with silver based filler metal
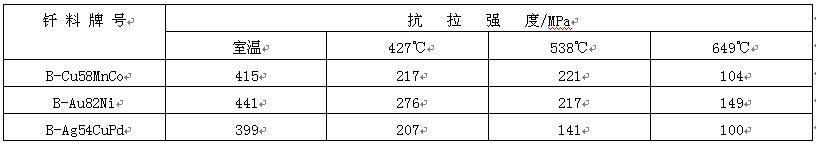
Copper based brazing filler metals used for brazing different steels are mainly pure copper, copper nickel and copper manganese cobalt brazing filler metals. Pure copper brazing filler metal is mainly used for brazing under gas protection or vacuum. The working temperature of stainless steel joint is not more than 400 ℃, but the joint has poor oxidation resistance. Copper nickel brazing filler metal is mainly used for flame brazing and induction brazing. The strength of the brazed 1Cr18Ni9Ti stainless steel joint is shown in Table 5. It can be seen that the joint has the same strength as the base metal, and the working temperature is high. Cu Mn co brazing filler metal is mainly used for brazing martensitic stainless steel in protective atmosphere. The joint strength and working temperature are comparable to those brazed with gold based filler metal. For example, the 1Cr13 stainless steel joint brazed with b-cu58mnco solder has the same performance as the same stainless steel joint brazed with b-au82ni solder (see Table 6), but the production cost is greatly reduced.
Table 5 shear strength of 1Cr18Ni9Ti stainless steel joint brazed with high temperature copper base filler metal
Table 6 shear strength of 1Cr13 stainless steel brazed joint
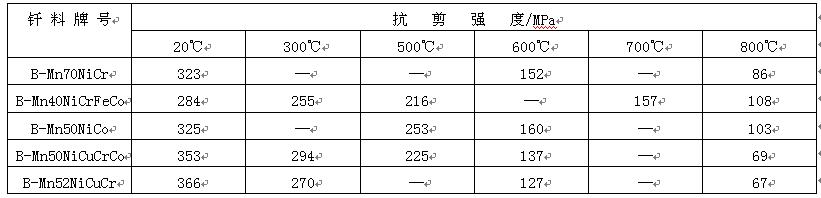
Manganese based brazing filler metals are mainly used for gas shielded brazing, and the purity of gas is required to be high. In order to avoid the grain growth of the base metal, the corresponding brazing filler metal with brazing temperature lower than 1150 ℃ should be selected. Satisfactory brazing effect can be obtained for stainless steel joints brazed with manganese based solder, as shown in Table 7. The working temperature of the joint can reach 600 ℃.
Table 7 shear strength of lcr18ni9fi stainless steel joint brazed with manganese based filler metal
When stainless steel is brazed with nickel base filler metal, the joint has good high temperature performance. This filler metal is generally used for gas shielded brazing or vacuum brazing. In order to overcome the problem that more brittle compounds are produced in the brazed joint during the joint formation, which seriously reduces the strength and plasticity of the joint, the joint gap should be minimized to ensure that the elements easy to form brittle phase in the solder are fully diffused into the base metal. In order to prevent the occurrence of base metal grain growth due to long holding time at brazing temperature, the process measures of short-time holding and diffusion treatment at lower temperature (compared with brazing temperature) after welding can be taken.
Noble metal brazing filler metals used for brazing stainless steel mainly include gold-based filler metals and palladium containing filler metals, of which the most typical are b-au82ni, b-ag54cupd and b-au82ni, which have good wettability. The brazed stainless steel joint has high high temperature strength and oxidation resistance, and the maximum working temperature can reach 800 ℃. B-ag54cupd has similar characteristics to b-au82ni and its price is low, so it tends to replace b-au82ni.
(2) The surface of stainless steel in flux and furnace atmosphere contains oxides such as Cr2O3 and TiO2, which can only be removed by using flux with strong activity. When stainless steel is brazed with tin lead solder, the suitable flux is phosphoric acid aqueous solution or zinc oxide hydrochloric acid solution. The activity time of phosphoric acid aqueous solution is short, so the brazing method of rapid heating must be adopted. Fb102, fb103 or fb104 fluxes can be used for brazing stainless steel with silver based filler metals. When brazing stainless steel with copper based filler metal, fb105 flux is used because of the high brazing temperature.
When brazing stainless steel in the furnace, vacuum atmosphere or protective atmosphere such as hydrogen, argon and decomposition ammonia are often used. During vacuum brazing, the vacuum pressure shall be lower than 10-2Pa. When brazing in a protective atmosphere, the dew point of the gas shall not be higher than -40 ℃ If the gas purity is not enough or the brazing temperature is not high, a small amount of gas brazing flux, such as boron trifluoride, can be added to the atmosphere.
2. Brazing technology
Stainless steel must be cleaned more strictly before brazing to remove any grease and oil film. It is better to braze immediately after cleaning.
Stainless steel brazing can adopt flame, induction and furnace medium heating methods. The furnace for brazing in the furnace must have a good temperature control system (the deviation of brazing temperature is required to be ± 6 ℃) and can be cooled quickly. When hydrogen is used as the shielding gas for brazing, the requirements for hydrogen depend on the brazing temperature and the composition of the base metal, that is, the lower the brazing temperature, the more the base metal contains stabilizer, and the lower the dew point of hydrogen is required. For example, for martensitic stainless steels such as 1Cr13 and cr17ni2t, when brazing at 1000 ℃, the dew point of hydrogen is required to be lower than -40 ℃; For 18-8 chromium nickel stainless steel without stabilizer, the dew point of hydrogen shall be lower than 25 ℃ during brazing at 1150 ℃; However, for 1Cr18Ni9Ti stainless steel containing titanium stabilizer, the hydrogen dew point must be lower than -40 ℃ when brazing at 1150 ℃. When brazing with argon protection, the purity of argon is required to be higher. If copper or nickel is plated on the surface of stainless steel, the requirement for the purity of shielding gas can be reduced. In order to ensure the removal of oxide film on the surface of stainless steel, BF3 gas flux can also be added, and lithium or boron containing self flux solder can also be used. When vacuum brazing stainless steel, the requirements for vacuum degree depend on the brazing temperature. With the increase of brazing temperature, the required vacuum can be reduced.
The main process of stainless steel after brazing is to clean the residual flux and residual flow inhibitor, and carry out post brazing heat treatment if necessary. Depending on the flux and brazing method used, residual flux can be washed with water, mechanically cleaned or chemically cleaned. If abrasive is used to clean the residual flux or oxide film in the heated area near the joint, sand or other non-metallic fine particles shall be used. Parts made of martensitic stainless steel and precipitation hardening stainless steel need heat treatment according to the special requirements of the material after brazing. Stainless steel joints brazed with Ni Cr B and Ni Cr Si filler metals are often treated with diffusion heat treatment after brazing to reduce the requirements for brazing gap and improve the microstructure and properties of the joints.
Post time: Jun-13-2022